CA-125
Noto anche come: CA-125 marcatore tumorale, Antigene carboidratico 125
Nome ufficiale: Antigene Tumorale 125
Ultima Revisione: 29.09.2020
Ultima Modifica: 24.08.2021
In Sintesi
Perché?
- Nelle pazienti con tumore dell’ovaio per la valutazione iniziale della neoplasia, per monitorare la risposta al trattamento e per evidenziare la comparsa di una eventuale recidiva.
- Nella valutazione di una massa pelvica sospetta, in associazione con l'ecografia.
- Nella sorveglianza delle donne ad alto rischio di cancro ovarico che scelgono di non eseguire la salpingo-ovariectomia bilaterale profilattica, in associazione con l'ecografia.
Non è invece raccomandato per lo screening di donne asintomatiche senza fattori di rischio genetici.
Quando?
- Nelle pazienti con carcinoma ovarico prima della chirurgia, prima dell’inizio della terapia e a intervalli regolari durante e dopo il trattamento.
- Nella fase di valutazione iniziale delle pazienti con una massa pelvica o che riferiscono sintomi sospetti.
- Periodicamente nella sorveglianza di donne con un rischio genetico aumentato a causa di mutazioni di geni BRCA e che non intendono eseguire la salpingo-ovariectomia profilattica.
Il campione
Un campione di sangue venoso.
La preparazione
Evitare di eseguire il prelievo durante il periodo mestruale.
L'Esame
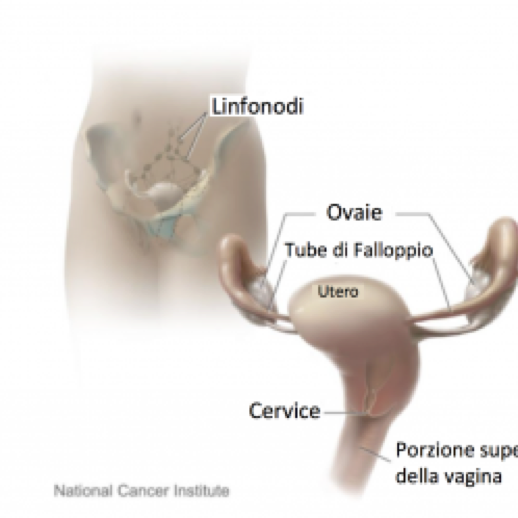
Questo esame misura la concentrazione dell'antigene carboidratico 125 (CA-125) nel sangue. Il CA-125 è una proteina presente sulla superficie cellulare in alcuni tipi di tumore e più raramente, in associazione con fatti infiammatori o altre patologie, anche in tessuti normali.
Il CA-125 è prodotto nella maggior parte (in circa l’80% dei casi) dei carcinomi sierosi dell’ovaio (i tumori più comuni di questo organo) e per tale ragione viene utilizzato come marcatore tumorale in questa neoplasia per monitorare l'efficacia del trattamento oppure per riconoscere una eventuale recidiva. Tuttavia, dato che circa il 20% dei tumori dell’ovaio non produce il CA- 125, alcune pazienti con carcinoma ovarico anche in stadio avanzato possono non avere livelli elevati di questo marcatore.
Il CA-125 può anche essere usato, in associazione con la visita clinica e gli esami ecografici, nella diagnosi differenziale di una tumefazione pelvica o nelle pazienti che riferiscono di avere con relativa continuità e frequenza (es. più di 12 volte al mese) uno dei seguenti sintomi: persistente distensione addominale (la paziente spesso riferisce questo sintomo come "gonfiore"), dolore pelvico o addominale persistente, aumento dell'urgenza e/o della frequenza di urinare).
Bisogna però tenere presente che il livelli di CA-125 nel sangue sono in relazione diretta con la estensione del tumore; quindi, in una paziente con un tumore iniziale i livelli del CA-125 nel sangue possono essere normali perché il tumore, essendo di piccole dimensioni, produce quantità limitate di marcatore.
Inoltre il CA-125 non è specifico per il carcinoma dell’ovaio in quanto può essere prodotto e rilasciato nel sangue anche da tessuti normali o infiammati e da altri tipi di tumore. I livelli nel sangue possono essere quindi moderatamente elevati in una varietà di condizioni oncologiche, non oncologiche e fisiologiche, comprese le mestruazioni, la gravidanza e le malattie infiammatorie pelviche.
A causa della bassa sensibilità per la malattia iniziale e della scarsa specificità tumorale il CA- 125 non è indicato per lo screening del carcinoma ovarico delle donne senza sintomi.
Anche nelle donne con varianti patogenetiche (mutazioni) nei geni BRCA1/2 associate a un aumentato rischio di cancro ovarico o che hanno una familiarità per questa malattia, lo screening di routine del cancro ovarico con il CA-125 e l'ecografia transvaginale non è raccomandato. L'ecografia transvaginale e/o la misurazione del CA-125 possono essere considerati solo per la sorveglianza a breve termine nelle donne ad alto rischio di cancro ovarico a partire dai 30-35 anni che scelgono di non eseguire la salpingo-ovariectomia bilaterale preventiva.
Sulla base dei dati raccolti dai Registri Tumori Italiani si è stimato che nel 2020 in Italia 5.180 donne abbiano avuto una diagnosi di tumore dell’ovaio e 3.000 siano decedute per questa malattia. Il cancro ovarico occupa in Italia il decimo posto per incidenza e il settimo per mortalità tra tutti i tumori nelle donne. L’elevata mortalità associata a questo tumore è principalmente dovuta a una sintomatologia non specifica e tardiva e alla mancanza di strategie di screening per effettuare una diagnosi precoce. Infatti, in circa l’80% dei casi la diagnosi viene fatta quando la malattia è in fase avanzata. In genere, il riscontro di un carcinoma iniziale ancora limitato alle ovaie o alla pelvi avviene occasionalmente durante controlli ginecologici di routine.
Pertanto l’identificazione di un test sensibile e specifico per la diagnosi precoce del carcinoma ovarico in donne asintomatiche rimane una priorità della ricerca.
Gli Intervalli di Riferimento – Cosa sono e come usarli
Per capire il significato clinico di un esame di laboratorio è necessario confrontare il proprio risultato con lo specifico intervallo di riferimento di quel tipo di esame. Gli intervalli di riferimento, chiamati a volte “valori normali”, indicano per ogni tipo di esame l’intervallo di valori che ci si
aspetta di trovare in una persona sana e sono in genere riportati sul referto di laboratorio accanto al risultato dello stesso.
Tuttavia, essendo i “marcatori tumorali” prevalentemente utilizzati a scopo di valutazione iniziale del paziente o di monitoraggio, e solo raramente nella diagnosi differenziale rispetto alla malattia benigna, gli intervalli di riferimento identificati come precedentemente descritto hanno solo un valore indicativo e non possono essere utilizzati per “classificare” il risultato dell’esame. Per questo è essenziale che il paziente si astenga dal tentativo di interpretare da solo il risultato dell’esame, e si raccomanda che, per l’interpretazione del risultato, il paziente si rivolga al medico curante che ha prescritto l’esame.
Nel caso del CA-125 bisogna anche ricordare che risultati e valori di riferimento possono variare da laboratorio a laboratorio se vengono utilizzati metodi diversi. É pertanto consigliabile eseguire il dosaggio di CA-125 nello stesso laboratorio e con lo stesso metodo per poter confrontare e interpretare correttamente i risultati durante il decorso della malattia.
Per maggiori informazioni si rimanda agli articoli: Gli Intervalli di Riferimento ed il loro Significato e Comprendere il Referto di Laboratorio.
Come e Perchè
Il CA-125 è utilizzato principalmente nel monitoraggio della risposta alla terapia del carcinoma dell’ovaio o per rilevare una eventuale recidiva del tumore dopo il completamento del trattamento. In queste situazioni cliniche una serie di prelievi raccolti in tempi successivi che mostrino livelli di CA-125 stabili, in aumento o in diminuzione è spesso più utile del risultato di un singolo prelievo.
Il CA-125 può essere anche utilizzato, sempre in associazione con la visita clinica e l’ecografia, nelle pazienti con una tumefazione pelvica o con sintomi sospetti per una patologia pelvica.
Tuttavia, il CA-125 non deve esser usato per lo screening del cancro ovarico nelle donne asintomatiche, perché non è un test specifico per il carcinoma ovarico. Infatti, i livelli di CA- 125 nel sangue possono essere moderatamente elevati in condizioni fisiologiche come la mestruazione, l’ovulazione e la gravidanza, in malattie non oncologiche sia della zona
pelvica (endometriosi, infiammazione pelvica, mioma uterino) che di altre parti del corpo (epatopatia, malattie flogistiche dell'intestino, ascite, versamento pleurico o pericardico, insufficienza cardiaca, polmonite, pancreatite, cirrosi epatica, epatite, malattie autoimmuni, insufficienza renale cronica) e in tumori di organi diversi dall’ovaio (pancreas, mammella, polmone, colon, mesotelioma, linfoma, timoma).
Il CA-125 può utilizzato assieme all'ecografia transvaginale per monitorare donne asintomatiche con un alto rischio di sviluppare un cancro ovarico, per facilitare una diagnosi precoce della malattia. Secondo le linee guida disponibili, questo uso del CA-125 dovrebbe essere però limitato alle donne che scelgono di non eseguire la salpingo-ovariectomia bilaterale profilattica.
Fattori di rischio per il carcinoma dell’ovaio sono la familiarità per questa malattia, l'avanzare dell’età, la storia riproduttiva e l'infertilità, l'obesità. Il fattore di rischio più significativo è rappresentato dall’essere portatori di mutazioni nei geni BRCA1 e BRCA2, che normalmente controllano la proliferazione cellulare e riparano i cromosomi danneggiati. Alcune alterazioni (mutazioni) di questi geni determinano col tempo una instabilità del genoma che può portare allo sviluppo di neoplasie. Quando una persona eredita da uno dei genitori una mutazione dei geni BRCA1 e/o BRCA2, ha quindi un rischio maggiore di sviluppare, nell’arco della propria vita, un tumore alla mammella e/o all’ovaio. Mutazioni di altri geni sono state associate a un aumentato rischio di carcinoma dell’ovaio e le pazienti con una condizione ereditaria chiamata sindrome di Lynch possono avere un rischio più elevato.
Meno del 20% dei tumori ovarici viene diagnosticato in fase iniziale, cioè prima che la malattia si estenda al di fuori dell'ovaio. Ciò è dovuto al fatto che in fase iniziale il carcinoma dell’ovaio è asintomatico o presenta sintomi aspecifici e generici. Anche se la ricerca è molto attiva nel campo della diagnosi precoce del tumore dell’ovaio, non esiste ancora oggi un metodo affidabile per la diagnosi precoce di questa malattia nelle donne asintomatiche. Pertanto, è opportuno ricordare l’utilità di eseguire regolarmente visite ginecologiche e essere consapevoli della storia familiare e di altri fattori di rischio.
Per ulteriori informazioni sul cancro ovarico, vedere la sezione " L’Esame" e l'articolo sul Tumore dell'ovaio.
Domande Frequenti
No, non tutti i tumori ovarici sono associati ad un aumento dei livelli di CA-125.
Innanzitutto, alcune tipologie di tumori più frequenti nelle donne giovani (es. tumori disgerminali) non rilasciano nel sangue quantità significative di CA-125, ma producono altri marcatori (es. HCG, AFP - Vedi le schede specifiche di questi marcatori).
Inoltre, circa il 20% dei classici carcinomi dell’ovaio di tipo più comune (tumori epiteliali sierosi) non produce CA125.
Infine, anche i carcinomi dell’ovaio che producono CA-125, quando sono in fase iniziale e sono perciò piccoli, rilasciano nel sangue limitate quantità di CA-125, così che livelli del marcatore possono rimanere normali. Nei carcinomi iniziali il livello del CA-125 nel sangue è infatti aumentato solo nel 40-60% dei casi.
Revisori
A cura di: Massimo Gion, Chiara Trevisiol, Aline S.C. Fabricio.
Documento revisionato e condiviso dai componenti attivi del Gruppo di Studio “Marcatori Tumorali” della Società Italiana di Biochimica Clinica e Biologia Molecolare Clinica (SIBioC): Emiliano Aroasio (Torino), Simone Barocci (Urbino), Ettore Capoluongo (Napoli), Giovanni Cigliana (Roma), Laura Conti (Roma), Mario Correale (Bari), Ruggero Dittadi (Mestre-Venezia), Antonio Fortunato (Ascoli Piceno), Fiorella Guadagni (Roma), Stefano Rapi (Firenze), Tiziana Rubeca (Firenze), Gian Luca Salvagno (Verona), Maria Teresa Sandri (Milano), Daniela Terracciano (Napoli), Alessandro Terreni (Firenze), Francesco Volpi (Aviano).
Centro Regionale Specializzato per i Biomarcatori Diagnostici Prognostici e Predittivi, Dipartimento di Patologia Clinica, Azienda ULSS 3 Serenissima – Ospedale SS. Giovanni e Paolo, Venezia. 2Istituto Oncologico Veneto IOV-IRCCS, Padova.
Immagini Correlate
Pagine Correlate
Altrove sul web
Fonti
AIRTUM Working Group. I tumori in Italia Rapporto 2019. Sopravvivenza. Epidemiol Prev 2018; 41(2):Suppl. 1. 3.
Associazione Italiana di Oncologia Medica (AIOM). Tumori dell’ovaio, edizione 2020. Associazione Italiana di Oncologia Medica: Milano, Italy; 2020. Available from https://www.aiom.it/linee-guida-aiom/. (Ultimo accesso 18 Maggio 2021).
Associazione Italiana di Oncologia Medica (AIOM). Tumori dell’utero, edizione 2020. Associazione Italiana di Oncologia Medica: Milano, Italy; 2020. Available from https://www.aiom.it/linee-guida-aiom/. (Ultimo accesso 18 Maggio 2021).
Colombo N, Creutzberg C, Amant F, et al.ESMO-ESGO-ESTRO Consensus Conference on Endometrial Cancer: diagnosis, treatment and follow-up. Ann Oncol. 2016 Jan;27(1):16-41. doi: 10.1093/annonc/mdv484
Colombo N, Sessa C, duBois A, et al. ESMO-ESGO consensus conference recommendations on ovarian cancer: pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease†. AnnOncol. 2019 May 1;30(5):672-705. doi: 10.1093/annonc/mdz062.
Committee Opinion No. 716: The Role of the Obstetrician-Gynecologist in the Early Detection of Epithelial Ovarian Cancer in Women at Average Risk. Obstet Gynecol. 2017 Sep;130(3):e146- e149. doi: 10.1097/AOG.0000000000002299.
Dodge J, Covens A, Lacchetti C, Elit L, et al. Management of a Suspicious Adnexal Mass. Program in Evidence-based Care Evidence-Based Series No.: 4-15 Version 2. Cancer Care Ontario: Toronto ON, Canada; 2016, September. Available from https://www.cancercareontario.ca/en/guidelines-advice/types-of-cancer/466.(Ultimo accesso 18
Maggio 2021).
Dunselman GA, Vermeulen N, Becker C, et al. ESHRE guideline: management of women with endometriosis. Hum Reprod. 2014 Mar;29(3):400-12. doi: 10.1093/humrep/det457.
Gion M, Trevisiol C, Rainato G, Fabricio ASC: Marcatori Circolanti in Oncologia. Guida all'Uso Clinico Appropriato. I Quaderni di Monitor AGENAS. Agenzia Nazionale per i Servizi Sanitari Regionali: Rome, Italy;2016. Available from https://www.agenas.gov.it/i-quaderni-di-monitor-
%E2%80%93-supplementi-alla-rivista/936-marcatori-circolanti-oncologia-guida-uso-clinico- appropriato. (Ultimo accesso 18 Maggio 2021).
Gruppo di Lavoro AIOM - SIGU - SIBIOC - SIAPEC-IAP. Raccomandazioni per l’implementazione del test BRCA nelle pazienti con carcinoma ovarico e nei familiari a rischio elevato di neoplasia. v2 -gennaio 2019. Available from /https://www.aiom.it/raccomandazioni- aiom-per-limplementazione-dell’analisi-mutazionale-brca-nei-pazienti-con-carcinoma-della- prostata-metastatico. (ultimo accesso 18 Maggio 2021).
Lancaster JM, Powell CB, Chen LM, Richardson DL; SGO Clinical Practice Committee. Society of Gynecologic Oncology statement on risk assessment for inherited gynecologic cancer predispositions. GynecolOncol. 2015 Jan;136(1):3-7. doi: 10.1016/j.ygyno.2014.09.009.
National Collaborating Centre for Cancer (UK). Ovarian Cancer: The Recognition and Initial Management of Ovarian Cancer. Cardiff (UK): National Collaborating Centre for Cancer (UK); 2011 Apr. Available from https://www.nice.org.uk/Guidance/CG122. (Ultimo accesso 18 Maggio 2021).
National Comprehensive Cancer Network (NCCN). Clinical Practice Guidelines in Oncology. Genetic/Familial High-Risk Assessment: Breast, Ovarian and Pancreatic Version 2.2021.
National Comprehensive Cancer Network: Fort Washington, PA, USA; 2020. Available from https://www.nccn.org/professionals/physician_gls/default.aspx. (ultimo accesso 18 Maggio 2021).
National Comprehensive Cancer Network (NCCN). Clinical Practice Guidelines in Oncology. Ovarian Cancer Including Fallopian Tube Cancer and Primary Peritoneal Cancer, Version 2.2018. National Comprehensive Cancer Network: Fort Washington, PA, USA; 2020. Available from https://www.nccn.org/professionals/physician_gls/default.aspx. (ultimo accesso 18 Maggio 2021).
National Comprehensive Cancer Network (NCCN). Clinical Practice Guidelines in Oncology. Uterine Neoplasms, Version 1.2021. National Comprehensive Cancer Network: Fort Washington, PA, USA; 2020. Available from https://www.nccn.org/professionals/physician_gls/default.aspx. (ultimo accesso 18 Maggio 2021).
National Collaborating Centre for Cancer (UK). Ovarian Cancer: The Recognition and Initial Management of Ovarian Cancer. Cardiff (UK): National Collaborating Centre for Cancer (UK); 2011 Apr. Available from https://www.nice.org.uk/Guidance/CG122. (Ultimo accesso 18 Maggio 2021).
Paluch-Shimon S, Cardoso F, Sessa C, et al. Prevention and screening in BRCA mutation carriers and other breast/ovarian hereditary cancer syndromes: ESMO Clinical Practice Guidelines for cancer prevention and screening. AnnOncol. 2016 Sep;27(suppl 5):v103-v110. doi: 10.1093/annonc/mdw327.
Practice Bulletin No. 149: Endometrial cancer. Obstet Gynecol. 2015 Apr;125(4):1006-1026. doi: 10.1097/01.AOG.0000462977.61229.de.
Practice Bulletin No 182: Hereditary Breast and Ovarian Cancer Syndrome. Obstet Gynecol. 2017 Sep;130(3):e110-e126. doi: 10.1097/AOG.0000000000002296.
Scottish Intercollegiate Guidelines Network (SIGN). Management of Epithelial Ovarian Cancer: A National Clinical Guideline (SIGN publication no. 135). SIGN: Edinburgh, UK; 2018. Available from http://www.sign.ac.uk. (ultimo accesso 18 Maggio 2021).
Sölétormos G, Duffy MJ, Othman Abu Hassan S, et al. Clinical Use of Cancer Biomarkers in Epithelial Ovarian Cancer: Updated Guidelines From the European Group on Tumor Markers. Int J Gynecol Cancer. 2016 Jan;26(1):43-51. doi: 10.1097/IGC.0000000000000586.
US Preventive Services Task Force, Grossman DC, Curry SJ, et al. Screening for Ovarian Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2018 Feb 13;319(6):588-594. doi: 10.1001/jama.2017.21926
Fonti utilizzate nelle precedenti revisioni
Thomas, Clayton L., Editor (1997). Taber's Cyclopedic Medical Dictionary. F.A. Davis Company, Philadelphia, PA [18th Edition].
Pagana, Kathleen D. & Pagana, Timothy J. (2001). Mosby's Diagnostic and Laboratory Test Reference 5th Edition: Mosby, Inc., Saint Louis, MO.
Pagana, K. D. & Pagana, T. J. (© 2007). Mosby's Diagnostic and Laboratory Test Reference 8th Edition: Mosby, Inc., Saint Louis, MO. Pp 219-220.
Wu, A. (© 2006). Tietz Clinical Guide to Laboratory Tests, 4th Edition: Saunders Elsevier, St. Louis, MO. Pp 210-211.
Mason, J. (Updated 2008 June 10). CA-125. MedlinePlus Medical Encyclopedia. [On-line information]. Available online at http://www.nlm.nih.gov/medlineplus/ency/article/007217.htm. Accessed March 2009.
(2007 March). Ovarian Cancer Resource Guide for women with recurrent disease. NOCC, Inc. [On-line information]. PDF available for download at http://www.ovarian.org/Images/ResourceGuideRecurrentOvarianCancer.pdf. Accessed March 2009.
(2008 August 26). Ovarian Cancer: Why Screening Isn't Routine. American Cancer Society. [On- line information]. Available online at http://www.cancer.org/docroot/SPC/content/SPC_1_ovarian_Q_A_Saslow.asp. Accessed March 2009.
(Modified 2008 April 3). Ovarian Cancer Screening (PDQ®), Health Professional Version. NCI,
U.S. National Institutes of Health. [On-line information]. Available online at http://www.cancer.gov/cancertopics/pdq/screening/ovarian/healthprofessional. Accessed March 2009.
(2008 July). Understanding CA 125 Levels: A Guide for Ovarian Cancer Patients. Gynecologic Cancer Foundation. [On-line information]. PDF available for download at http://www.thegcf.org/wcnlib/downloads/CA125levels_July2008.pdf. Accessed March 2009.
(Updated 2012 August 1). Fazal Hussain, F. and Homoud, H. Gynecologic Tumor Markers Tumor Marker Overview. Medscape Reference [On-line information]. Available online at http://emedicine.medscape.com/article/269839-overview. Accessed October 2012.
Zieve, D. and Chen Y. (Updated 2011 December 15). CA-125. MedlinePlus Medical Encyclopedia [On-line information]. Available online at http://www.nlm.nih.gov/medlineplus/ency/article/007217.htm. Accessed October 2012.
(Revised 2012 October 5). Can ovarian cancer be found early? American Cancer Society [On- line information]. Available online at http://www.cancer.org/cancer/ovariancancer/detailedguide/ovarian-cancer-detection. Accessed October 2012.
Grenache, D. et. al. (Updated 2012 May). Ovarian Cancer. ARUP Consult [On-line information]. Available online at http://www.arupconsult.com/Topics/OvarianCancer.html?client_ID=LTD. Accessed October 2012.
Schmidt, C. (2011 December 05). CA-125: A Biomarker Put to the Test. Medscape Today News from J Natl Cancer Inst. V 103(17):1290-1281 [On-line information]. Available online at http://www.medscape.com/viewarticle/750770. Accessed October 2012.
Rustin, G. et. al. (2012 March 08). CA-125: To Monitor or Not to Monitor? For Ovarian Cancer Patients in Remission. Medscape Today News [On-line information]. Available online at http://www.medscape.com/viewarticle/759809. Accessed October 2012.
(2012 September). Screening for Ovarian Cancer, U.S. Preventive Services Task Force Reaffirmation Recommendation Statement. USPSTF [On-line information]. Available online at http://www.uspreventiveservicestaskforce.org/uspstf12/ovarian/ovarcancerrs.htm. Accessed November 2012.
Pagana, K. D. & Pagana, T. J. (© 2011). Mosby's Diagnostic and Laboratory Test Reference 10th Edition: Mosby, Inc., Saint Louis, MO. Pp 210-211.
Clarke, W., Editor (© 2011). Contemporary Practice in Clinical Chemistry 2nd Edition: AACC Press, Washington, DC. Pp 499-500.
2016 review by Carolyn Sue Ziegler, MBA, MLS(CM).
Pagana, Kathleen D, Pagana, Timothy J, and Pagana, Theresa N. (©2015) Mosby’s Diagnostic & Laboratory Test Reference 12th Edition: Mosby, Inc. Saint Louis, MO. Pp.199-200.
(Updated Oct. 30, 2015) Gentry George T King, M.D. CA 125: Reference Range, Interpretation, Collection and Panels. Available online at http://emedicine.medscape.com/article/2087557- overview. Accessed 23/2016.
Ovarian Cancer National Alliance. Risk Factors. Available online at http://www.ovariancancer.org/about/risk-factors. Accessed 2/8/2016.
Ovarian Cancer National Alliance. Symptoms and Detection of Ovarian Cancer. Available online at http://www.ovariancancer.org/about/symptoms-of-ovarian-cancer-detection. Accessed 2/8/2016.
(Updated 13 January 2016) CA-125 blood test. MedlinePlus Medical Encyclopedia. Available online at https://www.nlm.nih.gov/medlineplus/ency/article/007217.htm. Accessed 2/3/2016.
(©2016) Mayo Clinic Mayo Medical Laboratories. Human Epididymis Protein 4, Serum. Available online at http://www.mayomedicallaboratories.com/test-catalog/Clinical+and+Interpretive/62137. Accessed 2/10/2016.
Ovarian Cancer Testing: HE4 Ovarian Cancer Monitoring. Quest Diagnostics. Available online at http://www.questdiagnostics.com/home/patients/tests-a-z/ovarian-cancer/he4-monitoring.html.
Accessed Feb 2016.
(©2014) Laboratory Corporation of America. A Technical Review, Human Epididymis Protein 4 (HE4), Monitoring Patients With Epithelial Ovarian Carcinoma.
(Jan 14, 2015) Fazal Hussain. Gynecologic Tumor Markers, Cancer Antigen 125. Medscape Review. Available online at http://emedicine.medscape.com/article/269839-overview#a2.
Accessed 2/8/2016.
(Aug 5, 2014) American Cancer Society. Ovarian Cancer: Detailed Guide. Available online at http://www.cancer.org/cancer/ovariancancer/detailedguide/ovarian-cancer-what-is-ovarian- cancer. Accessed 2/8/2016.

